TrialTwin :: Trial Designer
We're in the process of building metadata-driven, end-to-end Trial Designer and Simulator.
- Our goals are to have:
a single interface to all TrialTwin functional modules
allowing users to quickly define, build, load, and test studies
while allowing all roles across organization to "play" with studies
and to re-run study-level simulations at will, like a race car simulator
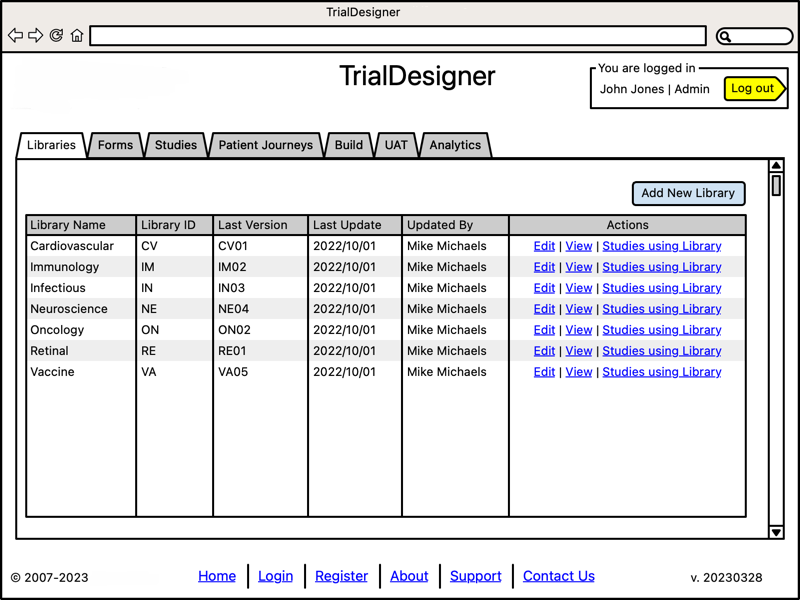
Manage Libraries
- Metadata-driven management:
Libraries by therapeutic area (see CRF Library Management)
Strict editing, role-based access control
Comprehensive version control
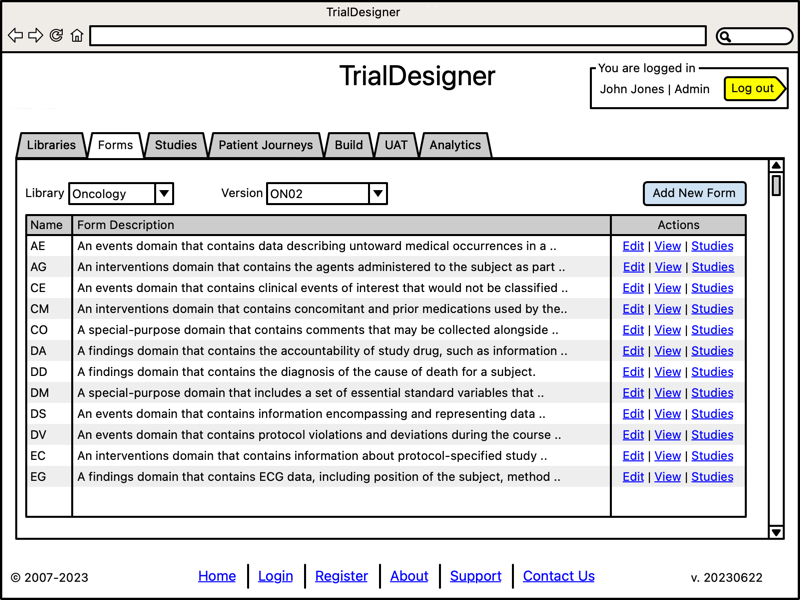
Manage Forms
- Form management:
Manage forms per library
Track studies using specific form
The image below shows the system pre-loaded with a series of forms defined by CDISC CDASH.
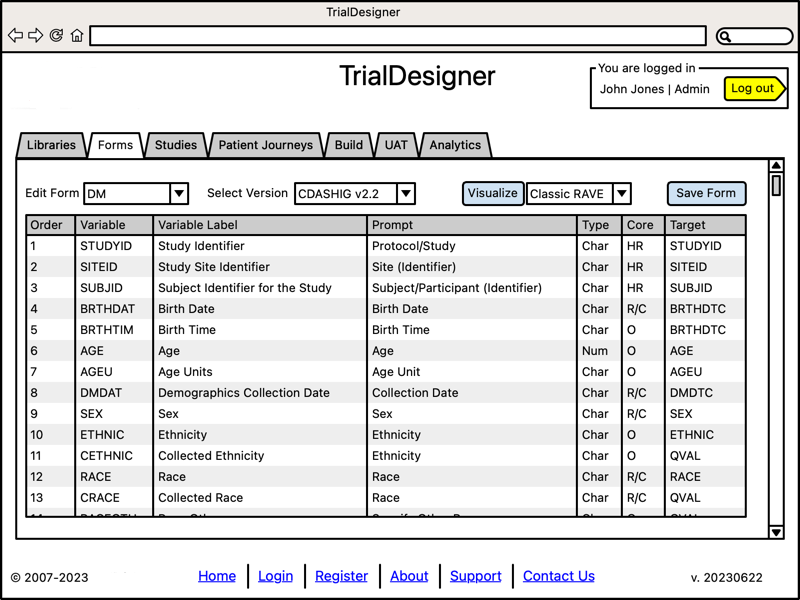
Edit Individual Forms
- Manage forms outside of an Electronic Data Capture ("EDC") system:
UI to create, edit, manage forms
EDC-agnostic
Visualize a form in several EDCs
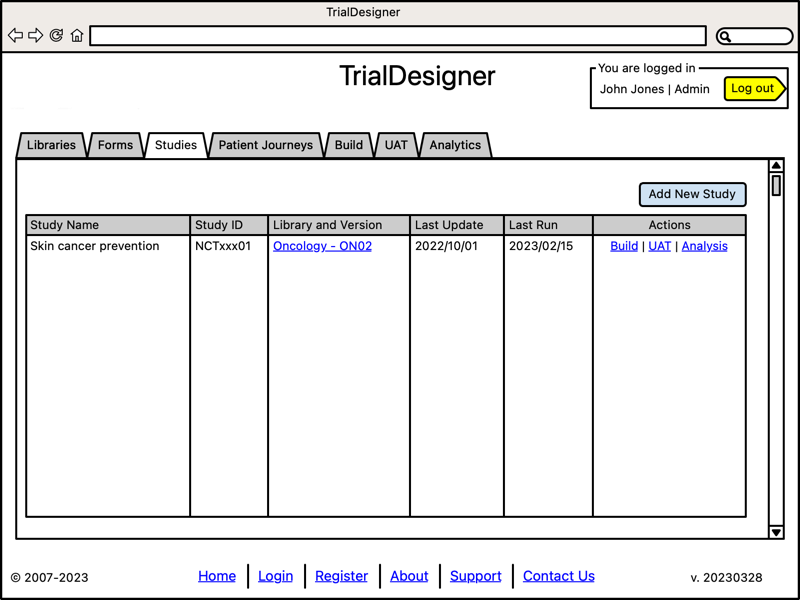
Manage Studies
- Centralized study management:
Assign to specific library version
- Centralized control center to:
build
run User Acceptance Testing
perform Analysis
System will allow users to create a new study by leveraging the existing libraries, CRFs within each library, and the inter-connected Controlled Terminologies, Biomedical Concepts, and terms stored in our Data Standards Governor platform.
Patient Journeys
- Define Patient Journeys:
By Therapeutic Area
By disease
Or for a specific study
User will be able to either re-use pre-defined Patient Journeys and/or create study-specific Patient Journeys.
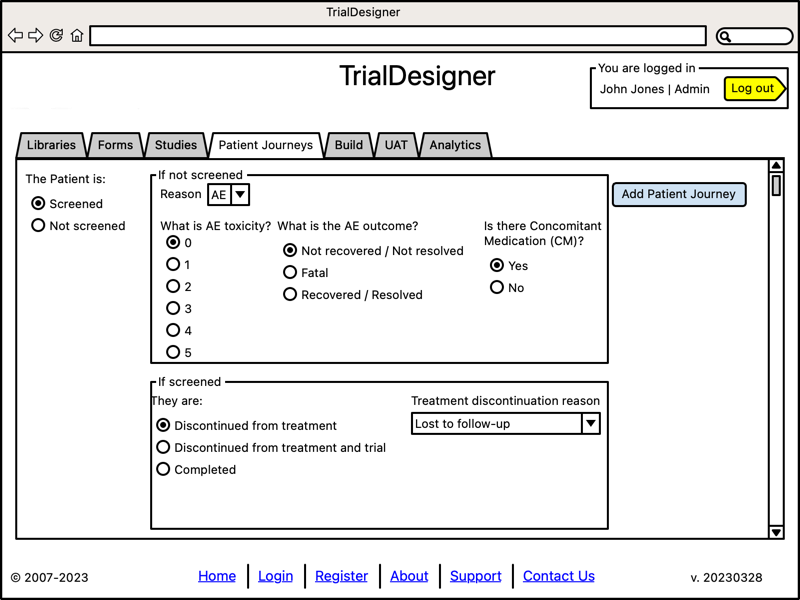
Build Study
- Build a study:
Define EDC to target
Choose to load test data
- Once a study is defined, TrialDesigner will interface with an EDC system to:
Build study using the metadata stored in the MDR
Load study-specific Synthetic Health Data (see Synthetic Health Data)
Run automated User Acceptance Testing ("UAT")
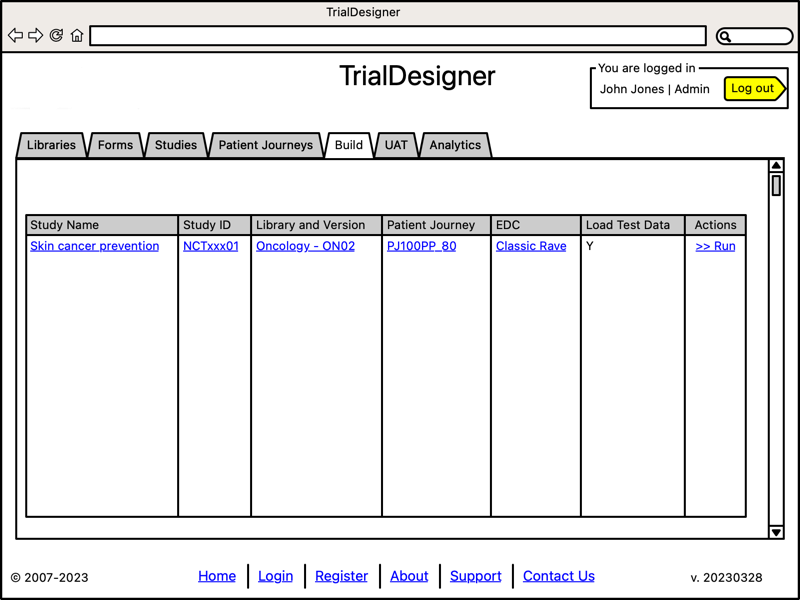
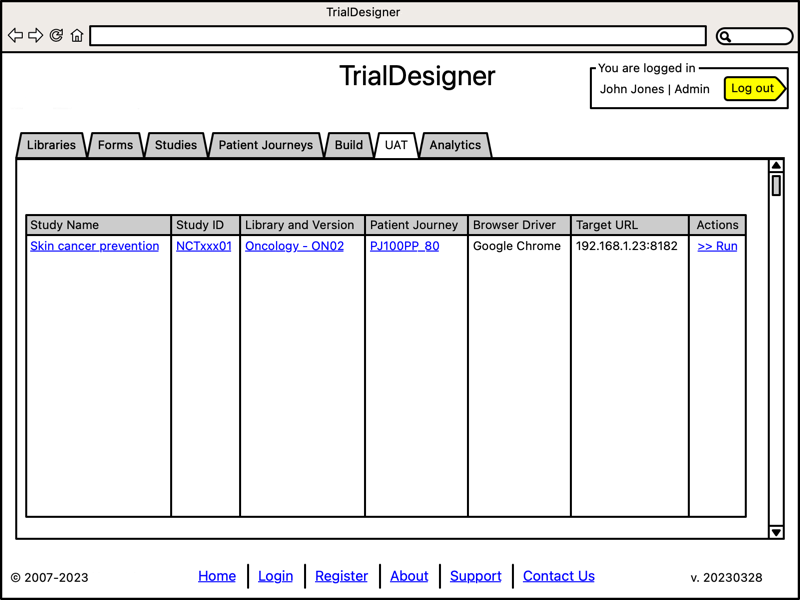
Run User Acceptance Testing
- User Acceptance Testing:
System runs automated UAT
Select browser type
Select target server (EDC)
TrialDesigner is designed to initially connect to Medidata RAVE.
This connection will be implemented as a "connector," allowing us to build other EDC-specific connectors.
- We re-use our own internal processes to demonstrate a metadata-driven UAT of each EDC. Where TrialDesigner:
automatically generates test scripts for Playwright1, a web-browser automation framework
run tests and generate reports, validation summaries
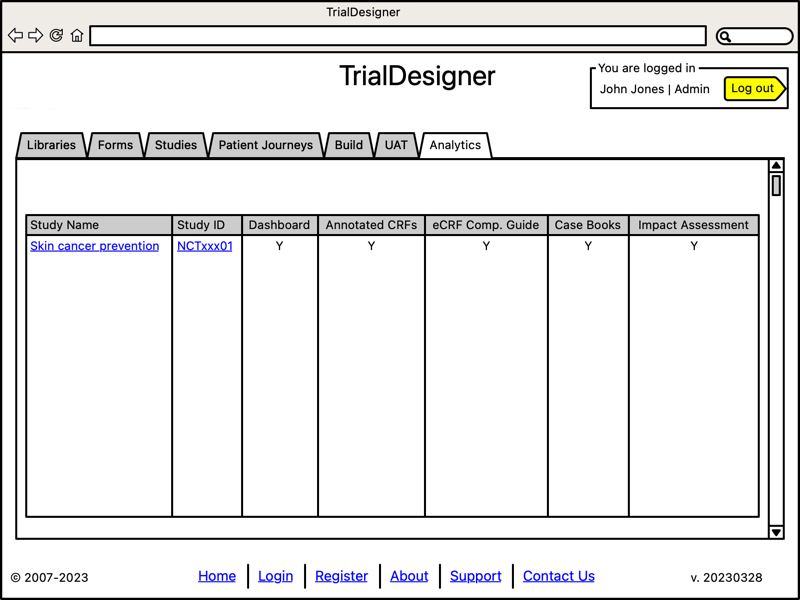
Analysis Phase
- Create Drafts for Analysis:
Dashboard
Annotated CRFs
eCRF Completion Guide
Case Books
Impact Assessment
Contact us
Please contact us for more details.