TrialTwin :: Our Vision
Our Point of View
We have a strong point of view of where the pharma industry can go in the next 10 years.
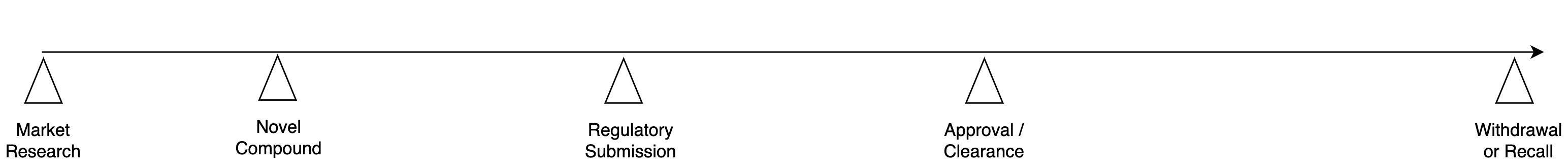
- First we outline the lifecycle of a medical device or pharma product:
Market Research: what disease / condition to target?
Novel Compound: substance with expected positive impact
Regulatory Submission: data submitted to seek clearance
Approval: regulatory clearance to market in target jurisdiction
Withdrawal / recall: end of the device's / drug's usable life
Our long-term goal is to use TrialTwin to manage both Drug Development Plans ("DDP") and Target Product Profiles ("TPP") for pharmaceutical companies.
- An Integrated Drug Development Plan ("DDP") for a new drug program:
improves efficiency
reduces costs
shortens timelines
increases probabilities of success
- And a Target Product Profile ("TPP") helps to coordinate the efforts of experts through the different phases of a new drug program:
Non-clinical
Clinical
Regulatory
Manufacturing
Commercial
Predicting Outcomes | 10-Year Vision
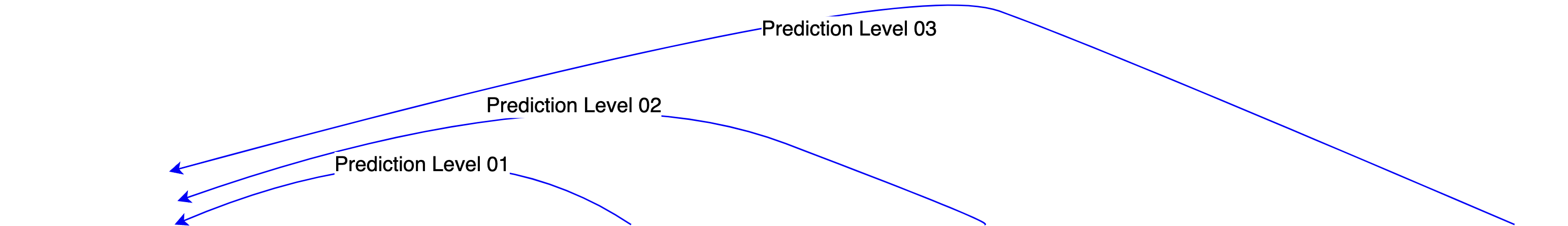

Prediction Level 01
Simulate the full regulatory submission package from the chemical structure of the new compound. * Historical data of chemical structures cleared earlier * Synthetic data of Subjects. Even before FPI.
Prediction Level 02
Simulate taking a new compound all the way out to regulatory approval. * Historical data of approval pathway followed by previous devices / drugs in similar class.
Prediction Level 03
Simulate the entire life-cycle of a new compound out to the marketing and revenue-producing stage. * Historical data of usage, reimbursement * Synthetic simulation of target population
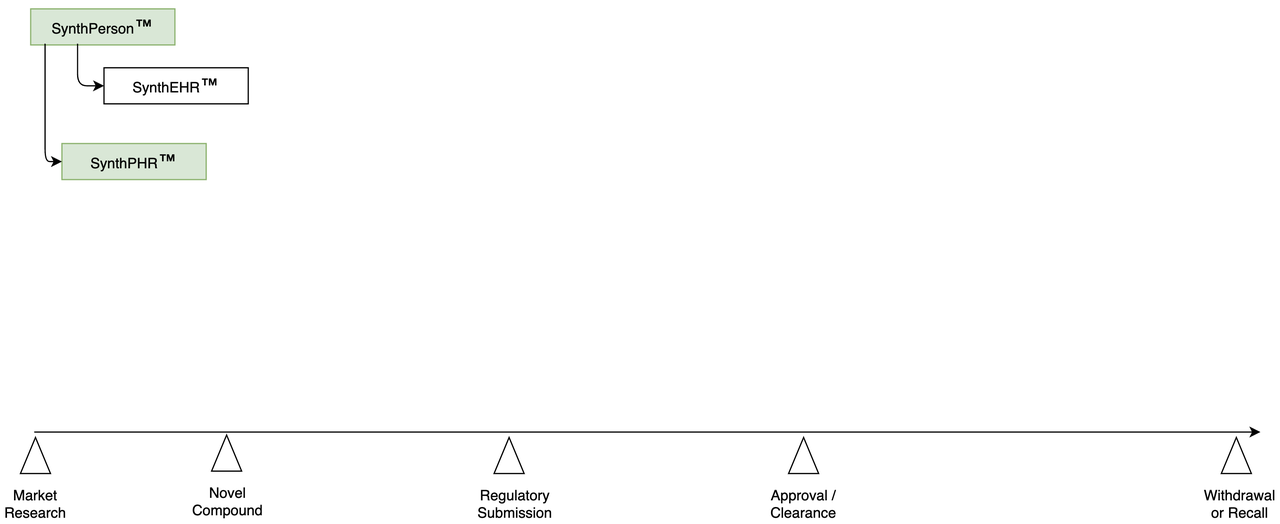
Phase I | Build a Synthetic Population
- Synthetic Population:
Create scientifically-accurate Synthetic Persons.
- Create full Synthetic Personal Health Record for each Person:
conditions
devices
drugs
lab results
Simulate hospital visits from Electronic Health Record ("EHR")
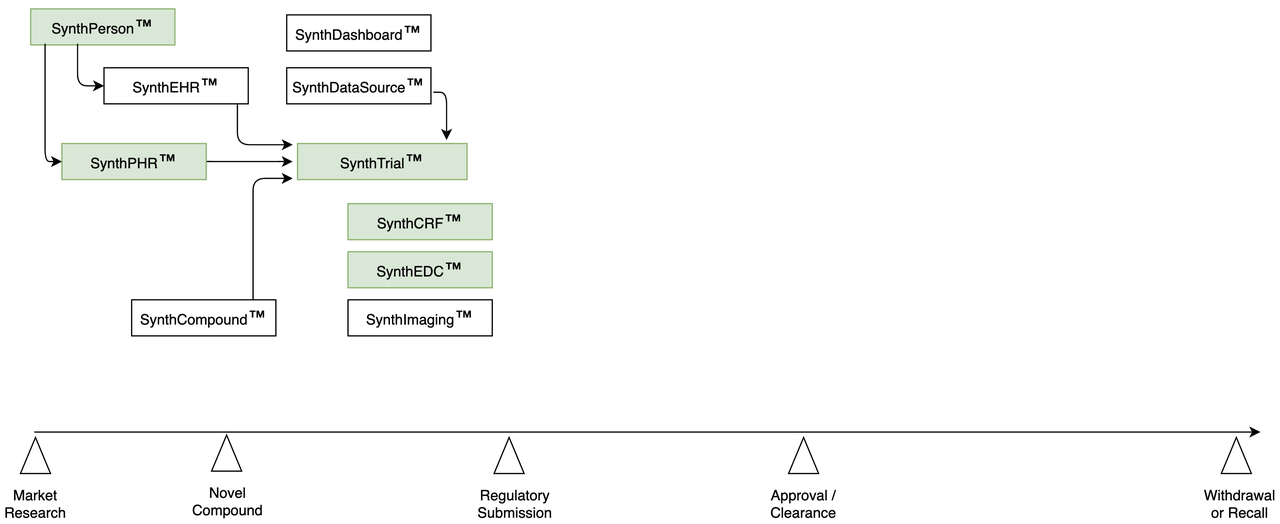
Phase II | Run a Synthetic Clinical Trial
- Start Data Management early:
Build study in EDC
With trial-specific parameters
Populate each CRF with realistic, fake synthetic data
Simulate RWD coming from non-CRF data sources
Phase III | Synthetic Submission
- Start analysis before FPI:
Build, test, and validate entire analysis process early
Prepare a test full submission with synthetic data
Generate all documents, reports, tables and figures
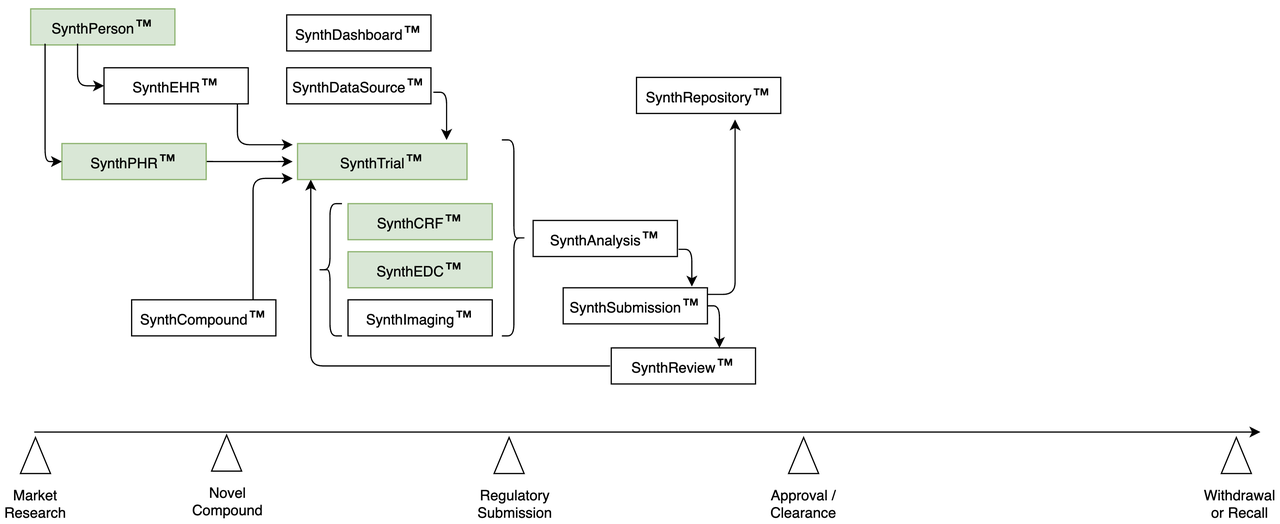
Accelerating Analysis | Trial Designer
- Integrated Simulation:
Build, test, and analyze each trial from one User Interface
Web-based tool avoids vendor lock-in, compatibility issues
Different roles, user access
Please see more details about our Trial Designer
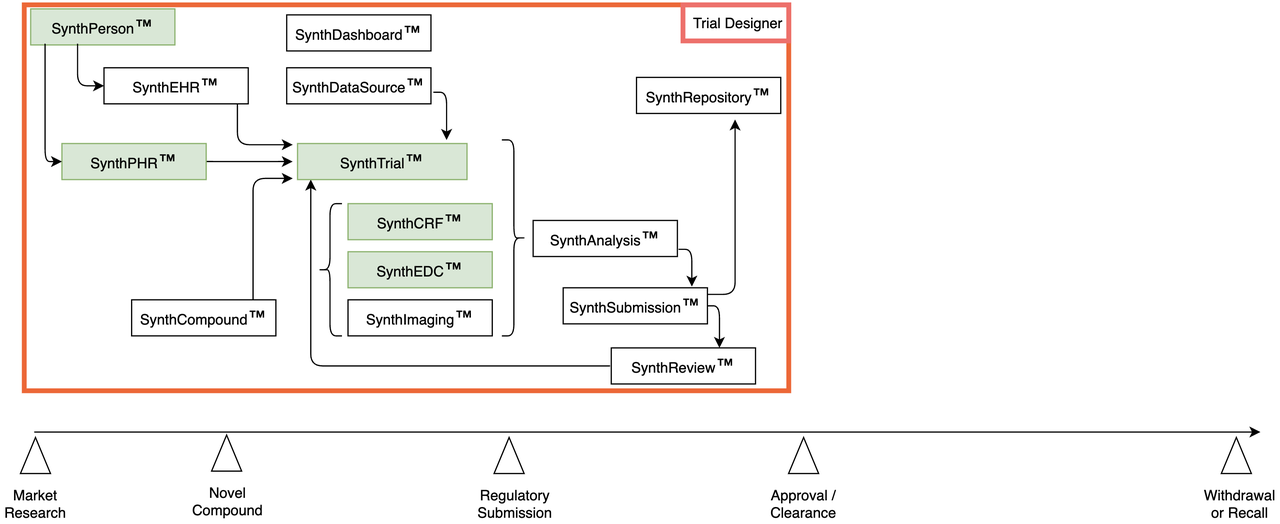
ClinicalTrials.gov | Synthetic Data for 38K Open Trials
- Each study includes:
Synthetic Subjects
Full medical history
Customized arms, visits
Full EDC data extract
All SDTM domains
Pre-built dashboards
Pre-generated reports
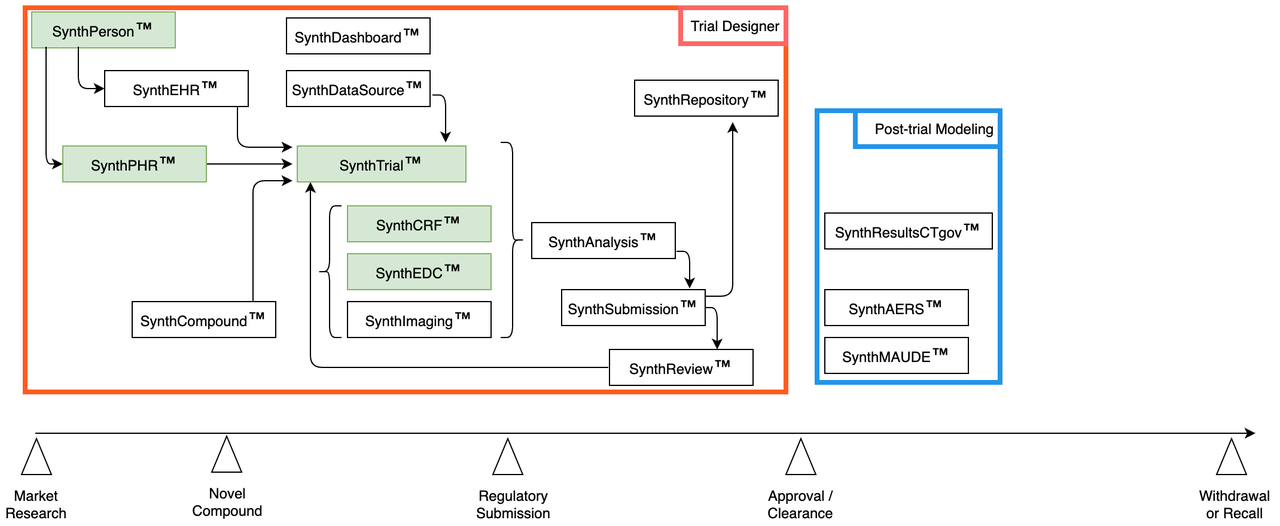
Phase IV | Post-trial Modeling
- Predict operating life:
Use historical data to predict adverse events: device, drug
Simulate uploading synth trial results to ClinicalTrials.gov
Phase V | Population Modeling
- Prescription patterns:
Use historical data to model marketing pathways
(US-specific) Medicare data
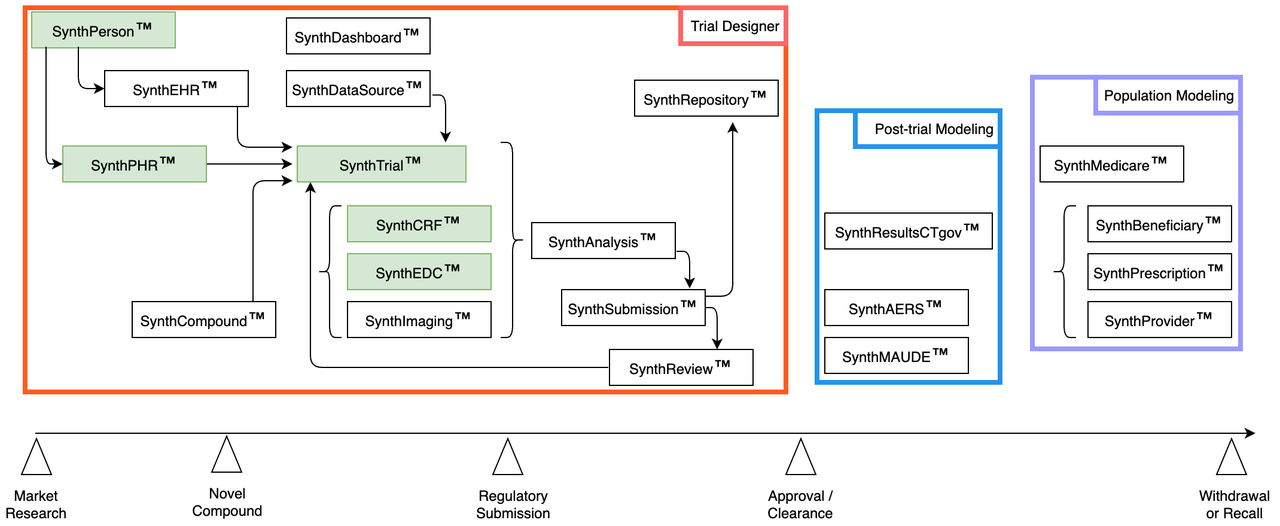
The historical data is sourced from our Regulatory Repository.
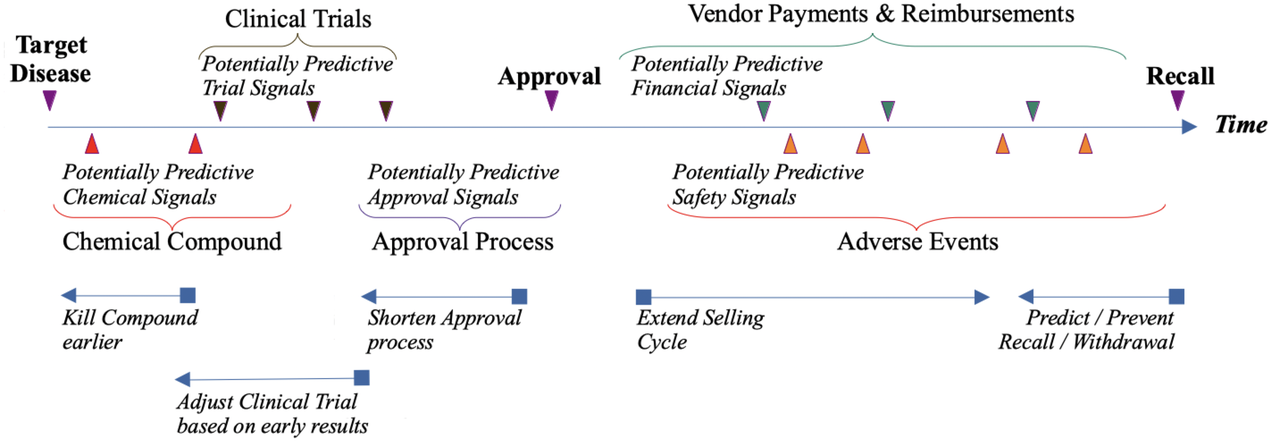
Prediction Model
In terms of direct business benefits, our prediction model could be useful to: * kill compound under study earlier * adjust clinical trial based on early results * shorten approval process * extend selling cycle * predict or prevent a drug's recall or withdrawal
Please see our Data Math concept.
And we presented our vision at the PHUSE EU Connect 2023 event with our Human BioGeography paper.
Contact us
Please contact us for more details.